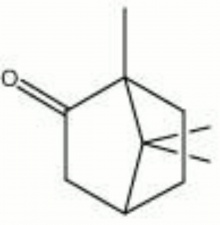
Determination Of The Molar Entropy Of Camphor (2)
 Camphor, C10H16O is an aromatic crystalline compound which is obtained from naturally from the wood or leaves of the camphor tree or synthesized. The camphor is employed in this experiment to determine its molar entropy of fusion. Molar entropy of fusion is denoted as ( ), which represents the entropy increases when a certain substance is melting. The entropy is usually depends on the state of the substance. The disorder in entropy of a substance is the lowest in solid state, whereas liquid is much more disorder and followed by gas state with highest disorder in entropy. In another words, the degree of disorder increases in the transition from a closely packed molecule arrangement solid to the disorganized molecules arrangement of liquid state. But, in this experiment, the entropy of camphor is the energy absorbed by the camphor is disturbed by the adding of naphthalene in the technique.
Camphor, C10H16O is an aromatic crystalline compound which is obtained from naturally from the wood or leaves of the camphor tree or synthesized. The camphor is employed in this experiment to determine its molar entropy of fusion. Molar entropy of fusion is denoted as ( ), which represents the entropy increases when a certain substance is melting. The entropy is usually depends on the state of the substance. The disorder in entropy of a substance is the lowest in solid state, whereas liquid is much more disorder and followed by gas state with highest disorder in entropy. In another words, the degree of disorder increases in the transition from a closely packed molecule arrangement solid to the disorganized molecules arrangement of liquid state. But, in this experiment, the entropy of camphor is the energy absorbed by the camphor is disturbed by the adding of naphthalene in the technique.
In modern day Arabic Zanjabiil means ginger, and it was most likely regarded a digestive aid. More importantly, even though, ginger was an exotic spice, desirable for flavoring undoubtedly it added a certain pungency to wine (Arabs created largely date wine, I think, although they had been by no signifies unfamiliar with Roman, Greek and Persian wines.
Pepper Spray – Roaches do not like red pepper. So, if you spray surfaces in your kitchen and bath with a answer containing red pepper, the will avoid these surfaces. An effortless recipe to whip up a batch of spray is to mix two tablespoons of Tobasco Sauce (the major ingredients are red pepper and vinegar) with 1 quart of water. Pour it into a pump spray bottle and mist it onto surfaces. A word of caution, you will be misting pepper spray into the air. Till the mist settles, you are most likely to …
Determination Of The Molar Entropy Of Camphor (2) Read More

 Smell owes its potency to the truth that, unlike other senses, it is straight connected with the emotion-creating regions in the brain. When you inhale an aroma, its molecules stimulate two tiny membranes deep in your nose. They stimulate receptors that trigger an electric signal to the limbic system and the hypothalamus. These ancient parts of the brain activate, handle, and integrate components of the nervous technique, endocrine technique, and several body functions like heart rate, respiration, temperature, blood sugar levels, waking, sleeping, and sexual arousal. They are also the seat of your most basic emotions-such as pleasure, anger, sadness, and worry-and are involved in memory.
Smell owes its potency to the truth that, unlike other senses, it is straight connected with the emotion-creating regions in the brain. When you inhale an aroma, its molecules stimulate two tiny membranes deep in your nose. They stimulate receptors that trigger an electric signal to the limbic system and the hypothalamus. These ancient parts of the brain activate, handle, and integrate components of the nervous technique, endocrine technique, and several body functions like heart rate, respiration, temperature, blood sugar levels, waking, sleeping, and sexual arousal. They are also the seat of your most basic emotions-such as pleasure, anger, sadness, and worry-and are involved in memory.